| energy-sources.html |   |
In previous articles 4. Use of wind energy, 5. Use of water gradient and 2. Sun radiation as source of energy are shown equipments and machines which can transform kinetic energy of wind, potential energy of water gradient and electromagnetic radiation to electricity or work. But there are equipments which can transform heat to work or to electricity. Inside these equipments are performed heat cycles(1). Why is possible this transformation of energy and essential terms are descripted in the article 43. Engineering thermomechanics. Parallely to the heat cycles which are transformed heat to work there are heat cycles which use work for increasing temperature of working fluid e.g. a refrigeration cycle.
In this article I describe only the most used heat cycles respectively only their ideal versions. The ideal heat cycle is composed from only reversible thermodynamic processes. Nevertheless these perfect cycles can not be performed in praxis only can be performed approximately (there are technical even investment limits) these cycles we can call real heat cycles. For example compare the Stirling cycle with a real cycle of the Stirling engine. The real cycle of the Stirling engine is function losses and use type of mechanism of piston, it is evident from real shape of Stirling engine cycle which is shown in article 36. Losses in Stirling engines. The real cycles are some similar the ideal cycles if their realization is very tardy and their losses are negligible. Therefore heat cycles which are composed only reversible thermodynamic process are called compared heat cycles. The main reason for reached high similarity the real cycle with ideal cycle is an increasing efficiency of heat cycle.
The heat cycles are drawn in p-V diagrams or in T-s diagrams, in which are better evidently of energy flows for calculation energy balance of cycle.
This term is used for cycles of piston machines, in which burning of fuel mix. These cycles are performed inside working volume – cylinder, therefore term internal combustion engine. The most frequently in technician world is can meet with modifications of three cycles: Lenoir cycle, Otto cycle and diesel cycle which are named by its inventors Jean Lenoir, Nikolaus Otto and Rudolf Diesel:
It is the cycle of gas reciprocating engine. The cycle is performed during one turn of the shaft at step by step the suction of air and flammable gas, the ignition and burning, expansion of hot exhaust gas and displaced of exhaust gas from the cylinder:
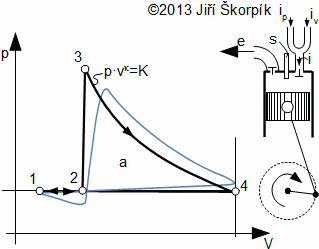
An ideal realization of the Lenoir cycle is composed by four reversible thermodynamic processes(2, 3, 4, 5):
The changes of states of the working fluid inside real engine are different from the changes of ideal cycle. These differences are caused by discontinuous motion of the piston and progress and speed of the burning.
This type of cycle is performed inside engines which are called Lenoir engines. Their first version contained a slide valve and as fuel has been used coal gas with atmospheric pressure which was mixed with air.
Because the heat is produced inside the Lenoir engine through exothermic chemical reaction during burning fuel mix is used term an internal combustion engine. Because at the exit cycle is changed the filling of the working gas inside the cylinder, so is used term for a open cycle. During calculation of the real Lenoir engine cycle is necessary take in account changes thermodynamic properties of the working gas during the burning.
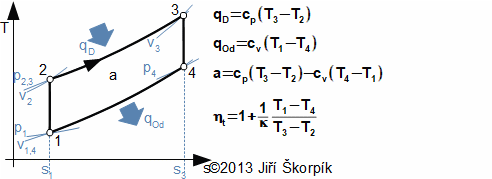
The ideal Otto cycle is composed by four reversible thermodynamic processes which are performed inside a cylinder with a piston and two valves (suction and exhaust valve). One cycle is performed during two strokes of the piston (four machine cycles). As working fluid is used a flammable mix usually the mix of air and vapor of fuel:
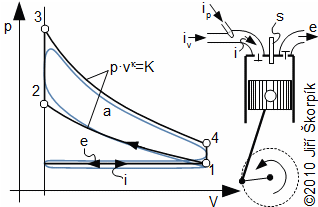
For ideal realization this cycle would have needed discontinuous movement of the piston, but this mechanism is not suitable for practical use and the move of the piston is performed continuously through a crankshaft mechanism. It means the isochoric processes on sections 1-3 and 4 – 1 can not be perfectly performed. In real situation the fuel mix is ignited in front of top dead position of the piston during compression (so called ignition timing in front of point 2), and the opening exhaust valve is done in front of the bottom dead position of the piston during expansion of the combustion gases. The real cycle are influenced also by the valve and shape suction and exhaust pipes, in which arises pressure drop, which decreases work of the cycle.
There are other realization where the cycle is apportioned only to two machine cycles. It means during motion of the piston to the bottom dead position proceeds gradually the combustion of fuel mix, the expansion and open of the exhaust valve and the exhaust. At motion of the piston to its top dead position are at start opened the exhaust and the suction valves. The suction of fuel mix is through a underpressure of the exhaust gas inside the cylinder. This underpressure is done at cooling of the exhaust gas. Following a closure of the suction valve and compression of the fuel mix. In front of the top position of the piston is ignited the fuel mix. In this case of two machine cycles is the exhaust valve localized at near the bottom dead position of the piston. These types of engines are simpler and have higher power output at the same speed, but usually worse the efficiency than four machine cycle engines.
The first realization this cycle was in Otto's engine for combustion liquid and gas fuels.
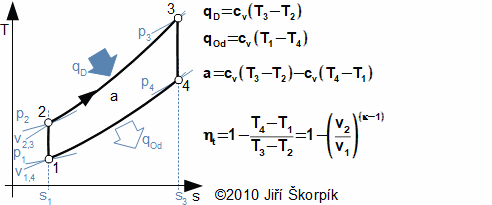
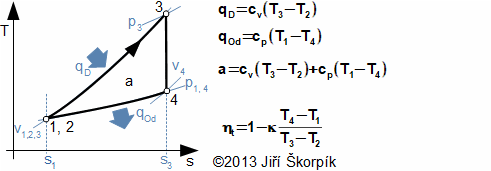
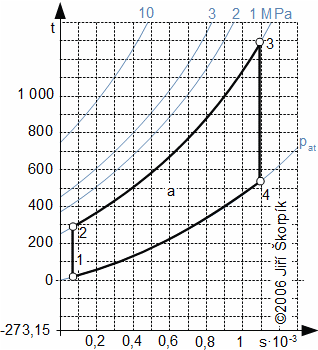
A Diesel cycle is divided on four machine cycles as the Otto cycle. Typical for the Diesel cycle is higher pressure ratio, isobaric heating of the working fluid and direct injection the fuel to the cylinder in front of the end of compression:
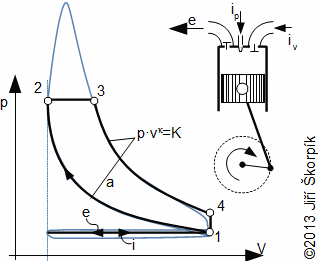 |
5.978 p-v diagram of Diesel cycle and its possible realization. The ideal realization of the cycle is drawn by the bold line. An approximate real realization of the Diesel cycle is drawn by dashed line. |
The suction of air of Diesel cycle proceeds at the motion of the piston to the bottom position and at open suction valve iv, the state 1. On the end of the suction is closed the suction valve and the isentropic compression of the air is started from state 1 to state 2. The pressure ratio is usually about 14 to 23. The fuel is injected through a nozzle ip at end of the compression of air. The state 2 of the fuel mix must match a state of self-ignition. Piston move to bottom dead position 3 must taken account a requirements on isobaric combustion. Between states 3-4 is performed isentropic expansion and the piston moves to the bottom dead position. The exhaust valve is opened at the bottom dead position (state 4), here the bigger portion of mass of the combustion gas is exhausted from the cylinder. The piston is without motion between 1-4.
The reciprocating motion of the pistons of majority the Diesel engines is transformed on rotary motion through a crank shaft as for case the Otto engines. This mechanism has influence on shape of the cycle and losses.
The two-stroke engine is simpler and has higher power output at the same speed rotation than the four-stroke engine, but it has lower efficiency outside nominal operation condition.
There are more types of the Diesel engines. For example a type with heating plug, which is placed in front of injection of the fuel mix , because for this type of engine must the fuel with air mixes in front of the inlet to the cylinder. If the fuel with low heat of combustion is used (e.g. biogas) then must be use even a spark plug.
Inside the Diesel engines is combusted the fuel mix at higher temperature than inside the Otto engines, therefore its internal efficiency is so higher. Disadvantage of high temperature is arise of harmful emissions NOx.
The Diesel engine has usually a supercharging (an increasing contents of air and fuel inside the cylinder) for increasing of its power output. The supercharging is usually performed by a turbocharger which is powered by the exhaust gas. For the supercharging of engines are used even other ways e.g.: a blower with mechanic drive through shaft of the engine or are used resonance suction tube and etc.
The supercharging is usually used at the Diesel engines, because the higher pressure at the suction is not problem. In case the Otto engines, the supercharging need not be always advantage, because the pressure and temperature at the end compression of the fuel mix must lower than is temperature of self-ignition. For this reason is the supercharging used at the Otto engines only for cases: the fuel has lower ignition temperature (e.g. aviation gasoline [2, s. 82]) – the density of air is lower (high altitude), for other cases is need decreasing pressure ratio of the engine then the supercharging may not be purposeful – the turbocharging can be used on Otto engines with a low cylinder compression ratio.
This cycle is the most widely used worldwide heat cycle in energy industry. The steam cycle is the most old heat cycle which is used in technical praxis and its history is descripted in the chapter 1. History of steam engines. As the working fluid is used water therefore it is colled "steam cycle", but is possible used alternative working fluids [25.]. For transformation heat to work through the steam cycle was used Steam piston engines, but in currently are usually used Steam turbinesteam turbines, but this fact is not change principle of the cycle. The steam turbine is a turbomachine which works continual way and the steam piston engine works discontinual (see chapter 11. Difference between piston engine and turbomachine). The steam cycle is performed through several equipments which are connected by pipes:
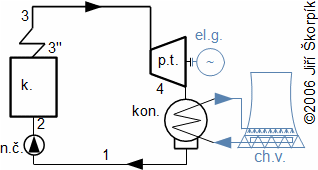 |
7.621 Simple flow chart of Rankine-Clausius cycle(6, 7, 8, 9). k. steam boiler (or steam generator of nuclear power plant; p.t. steam turbine or other type of steam engine; el.g. electric generator; kon. condenser (inside water steam is condensed); ch.v. cooling tower; n.č. feed pump (it increase pressure of water to boiler). |
The steam cycle which is drawn in T-s or i-s diagram gives real image about a way of transformation energy and energy flows:
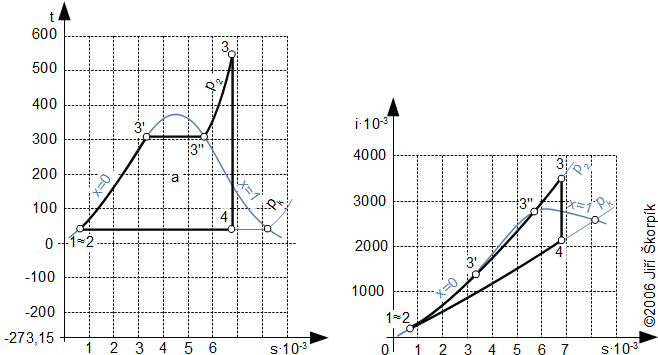
The heat flows to the working fluid is only in the boiler and the heat is rejected is performed only in the condenser. The turbine produces work, and the feed pump consumes work, therefore the work of cycle is a difference of these works:
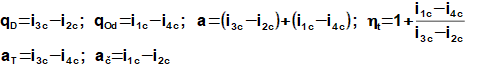
The steam cycle is the cycle with external heat transfer, therefore is possible use wide types of resources of heat (fossil fuels, biomass fuels, solar energy, nuclear energy etc).
The real flow chart of the steam cycle equipment does not look as simple as Figure 7. Due to the increase in performance and efficiency, heat recovery is performed, for heating and other purposes steam is taken from various parts of the cycle, including steam extraction in a steam turbine, see also Chapter 23. Steam turbines.
Brayton cycle is performed through several equipments. These equipments form a turboset with compression section (turbocompressor) and expansion section (turbine) and as the working fluid is gas. The cycle is usually performed as open cycle (an exchange of the working gas with surroundings), therefore this turboset is called as combustion turbine. The combustion turbines are wide used machines in airplane as a drive jet engine and in power industry. The combustion turbines are wide use machines in airplane as driver jet engine and in power industry.
In individual parts of the turboset are performed these thermodynamic changes in ideal case(10, 11, 12, 13):
The atmospheric air is sucked and compressed to pressure p2 by the compressor section for case the open cycle. The pressurized air is mixed with fuel (flammable fluid) at pressure p2 inside the combustion chamber, where it continuously burns. The exhaust gas are rejected to chimney at the end of the expansion.
 |
11.58 The Brayton cycle in T-s diagram of ideal gas. Base data sheet of this cycle: cp=1,004 J·kg-1·K-1, (dry air without CO2 at atmospheric conditions), κ=1,402, p1=pat (atmospheric pressure), p2=1 MPa, t1=20 °C, t3=1 300 °C, content of the working gas is stable. For case a combustion turbine is not content of the working gas stable due to burning inside the combustion chamber. This cycle was first performed by Georg Brayton (American) through piston engine. |
Energy balance of the Brayton cycle is as follows:
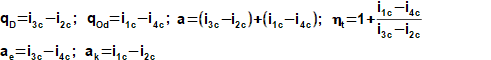
There are heat cycles, which transform of work on pressure energy, kinetic energy, potential energy or internal heat energy (e.g. refrigerators, heat pumps and etc.). Especially for case heat pump category is most used Vapor-compression refrigeration cycle. This cycle is similar to the Steam cycle but reverse order of changes of state quantities [3, p. 170], [5]:
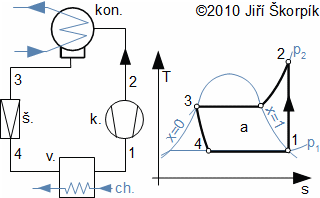
During ideal vapor-compression refrigeration cycle are carried out in the cycle these thermodynamic changes(14, 15, 16, 17):
Heat is delivery to the cycle through the evaporator and is rejected through the condenser. This type of cycle consumes work through compression:
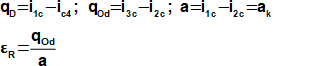
The refrigeration cycles are used for cooling or heating as heat pump. For case the cooling heat is taken from cool substance through an evaporator, which is usually located inside volume of cool substance or inside flow of cool substance.
For ideal cycles can be defined mean temperature of input heat of cycle T‾T and mean temperature of rejection heat of cycle T‾S. The mean temperature of input heat of cycle is equivalent of temperature at isothermal process between entropies smin and smax of solved heat cycle, where the amount input of heat at this isothermal process is the same as amount of input heat of solved heat cycle on this part of the cycle. The mean temperature of rejection heat of cycle is equivalent of temperature at isothermal process between entropies smax and smin of solved heat cycle, where the amount rejection of heat at this isothermal process is the same as amount of rejection heat of solved heat cycle on this part of the cycle:
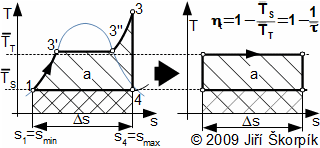
For example, for case the Carnot cycle is mean temperature of input heat of cycle equivalent to temperature T1 (T‾T=T1) and mean temperature of rejection heat of cycle equivalent to temperature T3 (T‾S=T3). At steam cycle are temperatures T‾T and T‾S is as follows:
Purpose this comparison is recovered possibilities of higher thermal efficiency cycles. The thermal efficiency is increasing with higher temperature ratio τ‾. It means that through increase the mean temperature of input heat or through decrease the mean temperature of rejection heat. For example, for case the steam cycle is evident that increasing pressure p2 is way to higher the mean temperature of input heat of cycle even to higher thermal efficiency regardless maximum temperature of the cycle. Similar way be can defined temperatures T‾T and T‾S for any heat cycle and through these temperatures is can assessed influence individual parameter of the working fluid on thermal efficiency. Process comparing a heat cycle with the Carnot cycle with purpose higher thermal efficiency is called Carnotization. For example, in energy industry is used Carnotization of steam cycle and Carnotization of Brayton cycle etc.
From compare the heat cycles with Carnot cycle is evident that for efficiency is most significant the temperature ratio τ‾ and not temperature ratio between maximum and minimum temperature of cycles.
By heat machine is called machine in which runs transformation internal energy and usually pressure energy of the working fluid on work or opposite(19, 20). It means heat exchangers (boilers, condensators and burners) are not the heat machines.

At present, it is being considered before building thermal power plant whether it could be replaced by a heating plant. The portion of heat must be rejected during transformation heat on work. This rejection heat can be use for heating (if near there is the heating consumer then it is can feed through a caliduct or a steam piping). The use of the rejection heat increase efficiency use energy in fuel. The CHP units must produce of heat in case a disorder on technology part for production electricity, because primary function of CHP units is production of heat. Temperature of working fluid in the caliduct and steam pipes must be on required levels (e.g. for heating and hot water for domestic 80 °C up 90 °C, for industry use higher), but an increasing mean temperature of rejection heat of heat cycle causes a decrease thermal efficiency of the cycle. For case the Power and heating plant to 2 MW power output is usually used term a Cogeneration unit. Characteristic parameters of CHP are a power to heat ratio and a cogeneration efficiency:

The power to heat ratio of CHP unit is function of type of heat machine and its power, for example the power to heat ratio CHP with steam cycle is from 0,15 to 0,4, CHP with Brayton cycle is from 0,4 to 0,7, CHP with internal combustion engines at higher power output is from 0,6 to 0,8 and CHP with combined cycle gas turbine is from 0,6 to 0,8 – The power to heat ratios other types of CHP units are shown chapter 10. Cogeneration in households.
The most use types of CHP unit in Czech republic are CHP units with internal combustion engines, CHP units with steam turbines and CHP units with combustion turbines. The CHP unit must contain an outlet of electric power even an outlet of heat for a heat consumers:
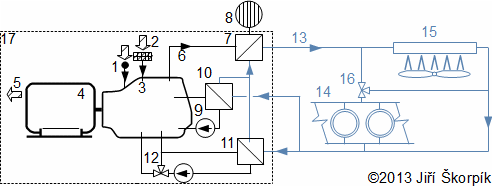
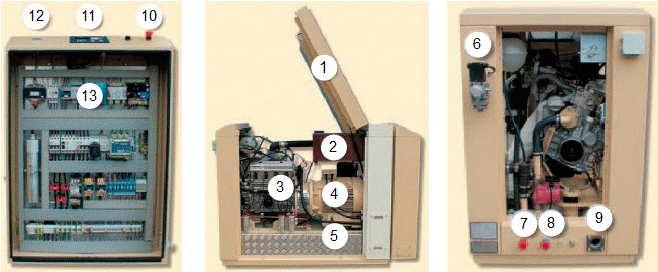
Small cogeneration unit with combustion engine are delivered in compact pack, but for cases bigger units is necessary building a machine room and an infrastructure:
This document is English version of the original in Czech language: ŠKORPÍK, Jiří. Tepelné oběhy a jejich realizace, Transformační technologie, 2006-11, [last updated 2015-10]. Brno: Jiří Škorpík, [on-line] pokračující zdroj, ISSN 1804-8293. Dostupné z https://www.transformacni-technologie.cz/06.html. English version: Heat cycles and their realizations. Web: https://www.transformacni-technologie.cz/en_06.html.